Lithium Assay “Liquid Stable Therapeutic Drug Monitoring Test”
Lithium Assay Liquid Stable Therapeutic Drug Monitoring Test
Improgen Lithium Assay kit is for quantitative in vitro determination of lithium in human serum. Measurements of Lithium are carried out essentially to ensure that proper drug dosage is administered in the treatment of patient suffering from bipolar disorder and to avoid toxicity.
ASSAY PRECISION
Improgen’s Liquid Stable Lithium Assay precision was evaluated according to NCCLS EP5-A guideline. Performance studies were conducted using the Beckman Coulter AU480 automated chemistry analyzer.
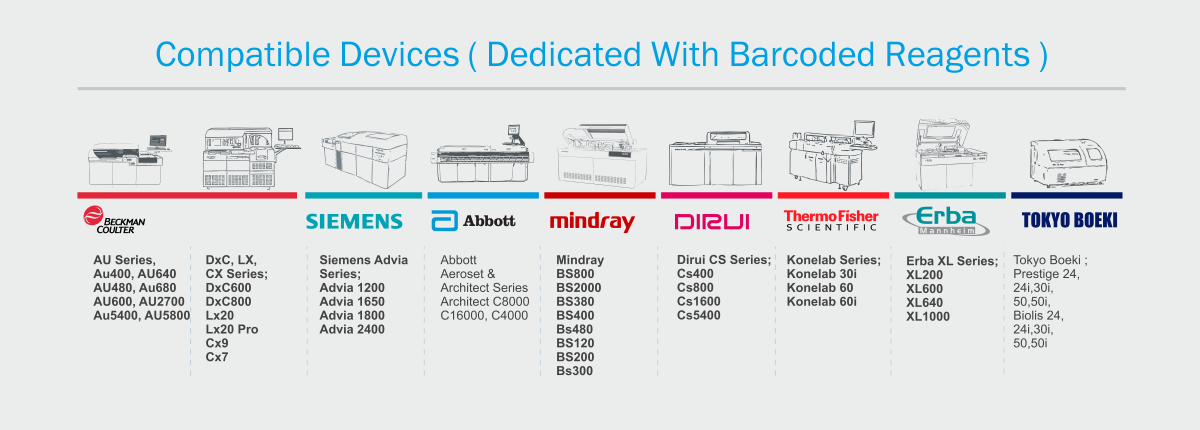
- Highly stable enzymatic method for accurate and precise test results
- Low sample volume vs. ISE methods
- Convenient liquid stable reagent set
- Traceable to NIST standards
- Not light sensitive
- Low cost per test
- Robust performance with excellent on-board reagent stability and calibration curve stability
- Neutral pH, not corrosive to instruments, and not hazardous to ship
- Wide range of instrument parameters available for simplifying implementation
- Excellent within run precision

